PALO ALTO, Calif., January 6, 2020 – Neumentum, Inc., a pharmaceutical company dedicated to transforming the way pain is treated – without opioids – today announced that Scott Shively, Chief Executive Officer, will present at the 2020 Biotech Showcase being held January 13-15, 2020 in San Francisco, CA. Mr. Shively will provide an overview of Neumentum, key clinical program updates, and highlights of a recently announced global licensing deal with Johnson & Johnson for the rights to novel oral analgesic products.
Presentation details are as follows:
Date: Monday, January 13, 2020
Location Hilton San Francisco Union Square – Franciscan D
Time: 10:45AM
The Biotech Showcase features public and private company presentations as well as plenary sessions and workshops that address a variety of business issues and therapeutic areas. It takes place during the course of the 38th Annual J.P. Morgan Healthcare Conference, one of the largest annual healthcare conferences that attracts investors and biopharmaceutical executives from around the world.
Neumentum: Addressing a National Health Emergency
Every day more than 1,000 people are treated in emergency rooms for misusing prescription opioids.[1]There were more than 17,000 deaths due to prescription opioid pain relievers in 2015, nearly three times the number of deaths in 1999.[2] Over 70 million surgical procedures are performed in the U.S. annually and postsurgical pain is routinely treated with opioid analgesics. America’s opioid crisis has been declared a national public health emergency.
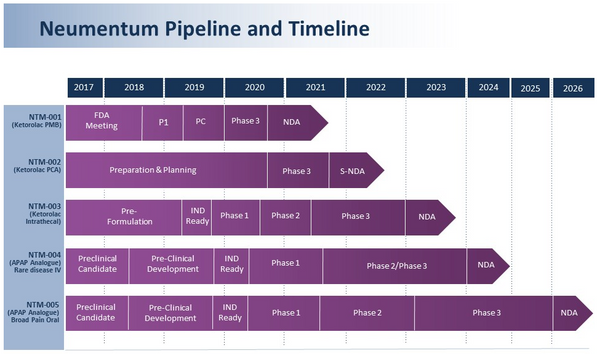
Neumentum is dedicated to becoming a leading non-opioid analgesic and neurology specialty pharmaceutical company with product candidates that have the potential to provide the benefits of safe and effective pain management without the limitations and risks for abuse and misuse that come with opioids. Neumentum’s lead product candidate, NTM-001 (novel, alcohol-free formulation of ketorolac in a pre-mixed bag for continuous IV infusion), has the potential to treat moderately severe acute pain that requires analgesia at the opioid level for up to 24 hours, usually in a postoperative setting, and to reduce the need for opioids. Additionally, JNJ-232, a product that is neither an opioid nor a NSAID, has completed positive Phase 2A clinical studies for moderate to moderately severe acute pain. Neumentum is led by a world-wide executive team of biotech and pharmaceutical industry leaders who have extensive pain and neurology experience, from drug development through commercialization. For more information, visit Neumentum.com.
[1] Centers for Disease Control and Prevention, Prescription Opioid Overdose Data. https://www.cdc.gov/drugoverdose/data/overdose.html
[2] National Institute on Drug Abuse, Overdose Death Rates. Supporting data. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates